Ensayo clínico: “Seguridad y efecto del ior®EPOCIM en pacientes convalecientes de infección por SARS-CoV-2 con secuelas cardiovasculares, renales y/o respiratorias”
miércoles, 6 de julio de 2022
Autor: CIM
Fuente: CIM
Fuente Exterior: MINSAP
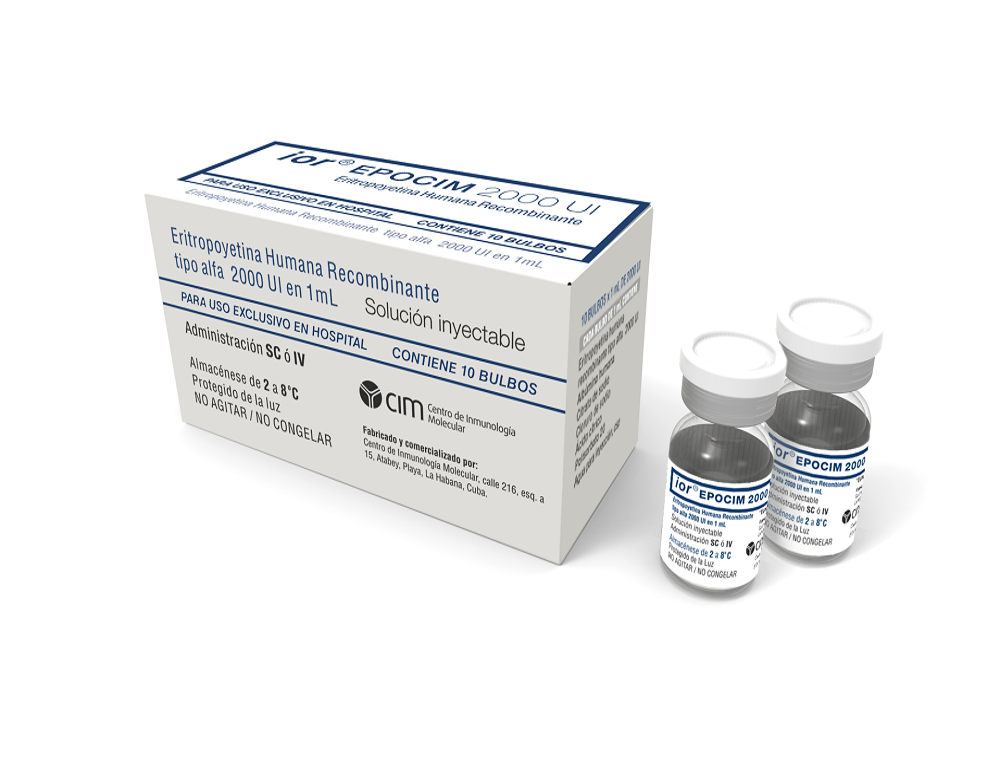 Se está realizando un estudio para evaluar la seguridad y el efecto del medicamento ior®EPOCIM (producido por el Centro de Inmunología Molecular), en el tratamiento de pacientes convalecientes post-COVID-19 con secuelas cardiovasculares, renales y respiratorias. Se podrán incluir sujetos cubanos de cualquier sexo, con edad igual o mayor de 19 años, que hayan padecido de COVID 19 (confirmada por PCR) y que 2 o 3 meses después y hasta el momento presenten manifestaciones como: Dolor en el pecho, ritmo cardíaco acelerado; Falta de aire (sobre todo a los esfuerzos), tos, dificultad al subir una escalera o loma, dificultad para caminar largas distancias; Cambios en la orina (orina espumosa o con sangre, más o menos cantidad de orina que la habitual o necesidad de levantarse en la noche para orinar), fatiga (se siente más cansado y tiene menos energía que la habitual), piel seca y le pica, hinchazón de manos o pies, bolsas alrededor de los ojos (sobre todo por la mañana), falta de aire, dolor en la parte baja de la espalda, hipertensión arterial, poco apetito o náuseas y vómitos, dificultad para concentrarse, problemas para dormir, calambres por la noche
Se está realizando un estudio para evaluar la seguridad y el efecto del medicamento ior®EPOCIM (producido por el Centro de Inmunología Molecular), en el tratamiento de pacientes convalecientes post-COVID-19 con secuelas cardiovasculares, renales y respiratorias. Se podrán incluir sujetos cubanos de cualquier sexo, con edad igual o mayor de 19 años, que hayan padecido de COVID 19 (confirmada por PCR) y que 2 o 3 meses después y hasta el momento presenten manifestaciones como: Dolor en el pecho, ritmo cardíaco acelerado; Falta de aire (sobre todo a los esfuerzos), tos, dificultad al subir una escalera o loma, dificultad para caminar largas distancias; Cambios en la orina (orina espumosa o con sangre, más o menos cantidad de orina que la habitual o necesidad de levantarse en la noche para orinar), fatiga (se siente más cansado y tiene menos energía que la habitual), piel seca y le pica, hinchazón de manos o pies, bolsas alrededor de los ojos (sobre todo por la mañana), falta de aire, dolor en la parte baja de la espalda, hipertensión arterial, poco apetito o náuseas y vómitos, dificultad para concentrarse, problemas para dormir, calambres por la noche
Se confirmará el diagnóstico de estas secuelas a través de tomografía axial computarizada u otras pruebas de funcionamiento cardiovascular o respiratorio, según indique el médico del estudio. No se podrán incluir mujeres embarazadas o en período de lactancia, o sujetos que presenten antecedentes de enfermedad tromboembólica en los últimos 3 a 6 meses, o antecedentes de enfermedades crónicas graves o que limiten la vida, o estén recibiendo tratamiento de diálisis o hemodiálisis, o hayan recibido tratamiento con algún producto en investigación en los últimos 6 meses. Tampoco se podrán incluir sujetos que presenten incapacidad mental que les impida entender la investigación, firmar el documento de consentimiento de participación y cumplir con el esquema de tratamiento y las evaluaciones previstas en el estudio.
Se conformarán dos grupos de tratamiento. Un grupo llamado “grupo control” recibirá tratamiento únicamente con la terapia convencional. Esta puede incluir fisioterapia respiratoria u otros fármacos que, según el criterio del médico puedan servir para el tratamiento de las secuelas cardiovasculares, renales o respiratorias en estudio. El otro grupo llamado “grupo estudio”, además de la terapia convencional recibirá 5 administraciones de ior®EPOCIM. La asignación de los sujetos a uno u otro grupo de tratamiento la realiza un personal que no participa en la investigación, por lo que todos los sujetos tendrán igual probabilidad de recibir uno u otro tratamiento.
Para mayor información dirigirse a:
Provincia Lugar donde se realiza la consulta del estudio (sitios ya en inclusión) Para contactar
La Habana Hospital 10 de Octubre
Bajos del Pabellón Mella en la Sala de Fisioterapia Lunes-8:30 am-12:00 m
Dra. Yulmys Rodríguez Borges, Consulta de Fisiatría,
Dr. Abdel del Busto Mesa
Telef: 78776267, ext 261
Pinar del Río Hospital Abel Santamaría 2do y 4to lunes: Dra. Yusleny Sánchez Orta, Neumología
3er miércoles. Dra. Yoima González Pérez, Consulta de Control 3, Hospital Abel Santamaría
Martes en la mañana: Dra. Arlety Pita Valdés, Consulta de Control 3, Hosp. Abel Santamaría
Teléf. 48 762068
Cienfuegos Hospital Gustavo Aldereguía Lima, CEA Martes, 8:00 am-12:00 m
Consulta general de evaluación, todos los días
Consulta de los EC:
Lic. Yuleidis García Rodríguez
Lic. Karelia Cortina Nápoles
Dr. Julio Jova Dueñas
Dra. Annia Hernández Marín
Telef: 43591751
Villa Clara Hospital Arnaldo Milián Lic. Margarita Ríos Cabrera
Dr. Ignacio Ramírez Gómez
Teléf: 42 270038
Camaguey Hospital Manuel Ascunce Domenech, Departamento de Ensayos Clínicos (en el sótano) Jueves- 9:00 am-12:00 m
Lic. Judith Ojeda de Pedro
Lic. Yaneidys Lores Méndez
Dra. Yanara Nélida Peláez Guevara
Telef: 32 253856
Holguín Hospital Vladimir Ilich Lenin,
Departamento de EC 1er y 3er jueves de cada mes, 9:00 am
Lic. Kenia Garcés Ávila
Dra. Irmary Riverón Proenza
Telef: 24 461601
Guantánamo Hospital Agostinho Neto
Consulta de Ensayos Clínicos Jueves- 1:00 pm-4:00 pm
Lic. Yadyleidis Elías Oquendo
Lic. Cecilia Sayoux Thaureaux
Dra. Anais González Jarrosay
Dra. Yanet Blanco Robles
Telef: 21 387319
Santiago de Cuba Policlínico de Especialidades adjunto al Hospital Saturnino Lora, Consulta de Ensayos Clínicos, 3er Piso, Puerta 6 Viernes, 9:00 am-12:00 m
Dr. Eduardo Rivero Rosales
Lic. Aimara Rosdo
Dr. Juan Carlos Sánchez Moráguez
Telef: 22 645447
Comentar
Comentarios
Preguntas Frecuentes
Cómo puedo acceder a las vacunas terapéuticas contra el cáncer de pulmón?
Cuba posee 2 vacunas terapéuticas para cáncer de pulmón avanzado:
CIMAvax EGF ® y VAXIRA® (racotumomab). Ambas vacunas terapéuticas tienen registro sanitario en la indicación de Cáncer avanzado de pulmón de células no pequeñas (CPCNP) estadio III y IV, después de la primera línea de quimioterapia. El principal beneficio clínico de estas vacunas se ha demostrado en un aumento de la supervivencia y mejoría de la calidad de vida, así como amplio perfil de seguridad.
¿Cómo adquirir las vacunas?
Los extranjeros deberán contactar a los Servicios Médicos de Cuba quienes evaluarán cada caso como parte de un programa integral de atención al paciente, con valor comercial.
-Servicios Médicos Cubanos. Web page:
www.smcsalud.cu.; E- mail: smc@smcsalud.cu
Teléf: (537) 209-0977 Fax: (537) 203-1590.
También puede hacer su solicitud de atención expresando el motivo de su solicitud, un resumen médico y datos personales directamente a:
Clínica Internacional del Hospital Hermanos Ameijeiras.
Calle San Lázaro # 701 esq. a Belascoaín,Centro Habana, La Habana, C.P:10400, Cuba.
Teléfono: (537) 8761029, (537) 8761030, (537) 8761683. Email: turismomedico@hha.sld.cu
Centro Internacional de Salud
La Pradera Calle 15 e/ 222 A y 234, Reparto Siboney, Municipio Playa, La Habana, Cuba
Teléfono: (+537) 273 7467 Ext. 403 (+537) 2737202. Email: comercia@cislapradera.cu
